SMART SERVICE PLATFORM FOR THE PHARMA & LIFE SCIENCES INDUSTRY
SMART SERVICE PLATFORM FOR MAXIMUM SAFETY & EFFICIENCY IN PHARMA AND LIFE SCIENCES INDUSTRY


MASTERING CHALLENGES IN A REGULATED ENVIRONMENT WITH OCULAVIS
Faster time-to-market & maximum productivity
Development period of pharmaceutical and medical technology products are often quite long before they receive all necessary approvals. At the same time, due to limited patent terms their time on the market is often limited. This places significant pressure on manufacturers, who want to swiftly realize the full production potential of their machines and equipment and to guarantee their long-term productivity.
Efficient service is essential to ensure maximum machine availability. The oculavis smart service platform empowers machinery and equipment manufacturers with AR-powered visual assistance so they can provide prompt global customer support via remote collaboration.

Differentiation in the market
To secure their market share in the pharma and life sciences industry machinery manufacturers must constantly differentiate themselves from competitors through innovations. To keep up with complex regulatory requirements and to thrive in a highly competitive environment the companies have to improve their processes with digital solutions.
With the oculavis smart service platform, machinery manufacturers can set themselves apart by enhancing their physical products with digital services. The solution not only facilitates a rapid reaction to customer requests along the entire machine life cycle, but also enables the introduction of digital business models.

Hohe Qualitätsstandards & Compliance
Stringent quality standards in the pharma and life sciences sector aim eliminating the risk of faulty products. Adhering to compliance regulations is crucial to maintaining consistently high quality in both production and testing processes.
oculavis SHARE enables close collaboration between pharma manufacturers and machinery suppliers, whose experts can quickly share their know-how via AR-powered visual assistance. Within the integrated case management system validation or qualification activities on machinery and equipment, as well as the documentation of machine incidents, can be done with ease.
Manufacturers of drug products use AR-powered visual assistance and digital work instructions for standard operating procedures (SOPs) to guarantee the accuracy of all process steps.

High service volumes
The service departments of pharma and life sciences equipment manufacturers handle a multitude of customer inquiries daily, ranging from spare parts quotations to on-site repair or retrofit consultations. Additionally, dealers and partners also often require assistance in resolving technical issues.
The oculavis smart service platform facilitates the efficient allocation of available personnel resources within the helpdesk and technical field service. Live support or self-service options for customers and partners help managing the high volume of service requests.

Datensicherheit & Datenschutz
Data security and compliance with data protection regulations are extremely important in pharmaceutical and life sciences processes. Due to the sensitive nature of data and confidential know-how involved in production, robust measures must be taken to protect them.
As the provider of the leading smart service platform, we place high demands on ourselves and our solution. oculavis is ISO 27001 certified. Our platform is securely hosted in the cloud on a European cloud server such as Microsoft Azure or Open Telekom Cloud (OTC), in full compliance with GDPR.
AR-powered visual assistance calls are end-to-end encrypted, while live anonymization of faces enhances employee data protection in production settings. Moreover, virtual walls in mixed reality can be used to shield confidential areas within laboratories and production facilities.


Faster time-to-market & maximum productivity
Development period of pharmaceutical and medical technology products are often quite long before they receive all necessary approvals. At the same time, due to limited patent terms their time on the market is often limited. This places significant pressure on manufacturers, who want to swiftly realize the full production potential of their machines and equipment and to guarantee their long-term productivity.
Efficient service is essential to ensure maximum machine availability. The oculavis smart service platform empowers machinery and equipment manufacturers with AR-powered visual assistance so they can provide prompt global customer support via remote collaboration.

Differentiation in the market
To secure their market share in the pharma and life sciences industry machinery manufacturers must constantly differentiate themselves from competitors through innovations. To keep up with complex regulatory requirements and to thrive in a highly competitive environment the companies have to improve their processes with digital solutions.
With the oculavis smart service platform, machinery manufacturers can set themselves apart by enhancing their physical products with digital services. The solution not only facilitates a rapid reaction to customer requests along the entire machine life cycle, but also enables the introduction of digital business models.

High quality standards & compliance
Stringent quality standards in the pharma and life sciences sector aim eliminating the risk of faulty products. Adhering to compliance regulations is crucial to maintaining consistently high quality in both production and testing processes.
oculavis SHARE enables close collaboration between pharma manufacturers and machinery suppliers, whose experts can quickly share their know-how via AR-powered visual assistance. Within the integrated case management system validation or qualification activities on machinery and equipment, as well as the documentation of machine incidents, can be done with ease.
Manufacturers of drug products use AR-powered visual assistance and digital work instructions for standard operating procedures (SOPs) to guarantee the accuracy of all process steps.

High service volumes
The service departments of pharma and life sciences equipment manufacturers handle a multitude of customer inquiries daily, ranging from spare parts quotations to on-site repair or retrofit consultations. Additionally, dealers and partners also often require assistance in resolving technical issues.
The oculavis smart service platform facilitates the efficient allocation of available personnel resources within the helpdesk and technical field service. Live support or self-service options for customers and partners help managing the high volume of service requests.

Data security & protection
Data security and compliance with data protection regulations are extremely important in pharmaceutical and life sciences processes. Due to the sensitive nature of data and confidential know-how involved in production, robust measures must be taken to protect them.
As the provider of the leading smart service platform, we place high demands on ourselves and our solution. oculavis is ISO 27001 certified. Our platform is securely hosted in the cloud on a European cloud server such as Microsoft Azure or Open Telekom Cloud (OTC), in full compliance with GDPR.
AR-powered visual assistance calls are end-to-end encrypted, while live anonymization of faces enhances employee data protection in production settings. Moreover, virtual walls in mixed reality can be used to shield confidential areas within laboratories and production facilities.
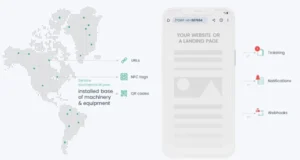
OCULAVIS PLATFORM FEATURES FOR INTEGRATED PHARMA AND LIFE SCIENCES PROCESSES
Integration options
With the oculavis API or standard connectors, the platform integrates seamlessly into existing process landscapes and third-party systems.
Live collaboration with AR annotations
Pharma and life sciences experts collaborate completely digitally. AR annotations provide precise instructions for remote commissioning, troubleshooting, training, inspections and audits.
Cases for ticketing & documentation
The integrated cases module can be used as a comprehensive and adaptable ticket system. It facilitates seamless, structured, and traceable documentation of regulated processes.
Management of the installed machine base
In oculavis SHARE you can define authorizations on a functional level, create structures for mapping service organizations and to assign experts (teams) to assets.
Digital work instructions for standardized procedures
Digital work instructions can be used for standard operating procedures (SOPs). They ensure that procedures are executed and documented accurately in the appropriate order.
White label options
The oculavis SHARE platform offers white label options for full customization to match the corporate identity of pharma and life sciences companies.
SECURE AND SCALABLE IN A REGULATED INDUSTRY
Seamless integration into the existing PDM and PLM systems.
Performing maintenance, safety inspections, metrological checks, functional tests, and quality controls remotely.
Speed up the processing time of inquiries and efficiently manage the high volume of service requests.
Minimize downtime and lower the environmental impact by reducing travel.
GLOBAL TECHNOLOGY GROUP RELIES ON REAL-TIME SUPPORT


Reduction of machine downtimes by up to 70 %.
Optimization of process quality during commissioning (SAT), maintenance, troubleshooting, and spare parts replacement.
Immediate support for end customers on the shop floor with precise instructions via Augmented Reality annotations.
PHARMA AND LIFE SCIENCES COMPANIES THAT HAVE OPTIMIZED THEIR PROCESSES WITH OCULAVIS




OCULAVIS IS YOUR RELIABLE PARTNER IN THE PHARMA AND LIFE SCIENCES INDUSTRY
Arrange your meeting with our experts. We look forward to exchanging ideas!
DISCOVER INNOVATIVE PROCESSES

Success factors and regulatory framework for the introduction of AR video support in the pharmaceutical industry